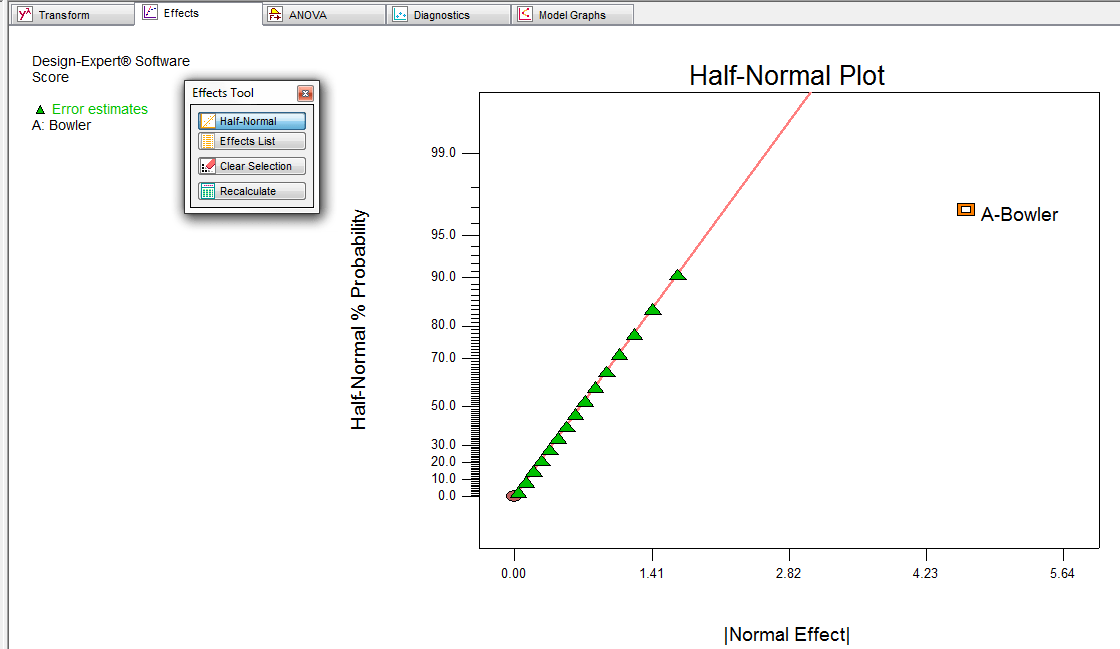
Figure 7 c represented the observed response values compared with that of the predicted values depicting a good fit. In this case, effect of A and B was significant. A predominant effect of factor A was visualized from the equation that with increase in concentration of buccoadhesive polymer A , the swelling property of the wafer also increases. Composition of the casting solution as per experimental design to prepare wafer formulation FNA 1 trial number 5. The process was optimized for the dependent response variables Y 1 to Y 4 , and the optimized formula was reached by keeping the bioadhesion force in target of 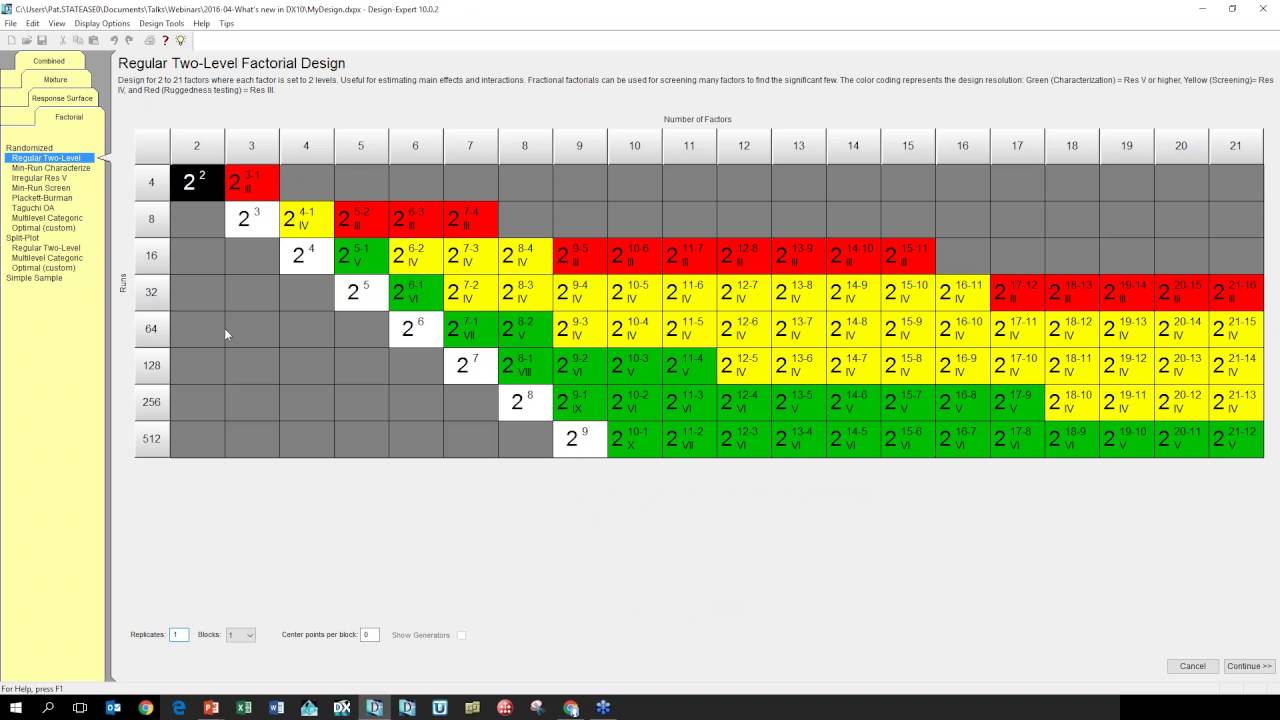
| Uploader: | Groll |
| Date Added: | 17 March 2015 |
| File Size: | 21.63 Mb |
| Operating Systems: | Windows NT/2000/XP/2003/2003/7/8/10 MacOS 10/X |
| Downloads: | 12254 |
| Price: | Free* [*Free Regsitration Required] |
Characterization and optimization of orodispersible mosapride film formulations. The aim of RSM is to discover the optimum operating conditions for a given system or the way in which a particular response is modified by a set of variables over expedt specific regions of interest.
Received Apr 4; Accepted May 2.
Design–Expert - Wikipedia
The measurement was made in triplicate with the standard deviation as a measure of variation. RSM provides an estimate for the value of responses for every possible combination of the factors by varying the values of all factors in parallel, making it experg to comprehend a multi-dimensional surface with non-linear shapes.
X-Ray powder diffraction pattern of Loratadine, blank wafer, and drug-loaded wafer formulation FNA 6. Views Read Edit View history.
The process was optimized for the dependent response variables Y 1 to Y 4and the optimized formula was reached by keeping the bioadhesion force in target of A linear relationship was observed between response Y 2 and factor A and B. Swelling is an important parameter to be studied before considering bioadhesion [ 23 ].
In this case, ABA 2and B 2 were significant. The in vitro drug release of the wafers was determined using a paddle type dissolution apparatus Excel Enterprises, Kolkata, India.
Experimental Design A 3 2 factorial design was employed where the amount of two carriers factors were varied at three levels as hypothesized by the design. The pharmaceutical wafers hold potential advantages like rapid disintegration, no swallowing or chewing, no coadministration of water, accurate dosing compared to liquid products, great safety, and efficacy along with patient compliance.
The disintegration time of the wafer increased proportionately with the fraction of sodium alginate and lactose monohydrate. This supports the process of optimization by rendering an empirical model equation for the response as a function of the different variables. The combined effect of factors A and B could be explicated with the help of response surface and contour plots Figures 9 a and 9 b.
An optimum .80.7.1 for the formulation was generated by the numerical optimization technique following desirability approach. Disintegration Study [ 16 ] The wafer size required for dose delivery 3. Factorial designs, dealing ttial factors in all possible combinations, are considered to be the most efficient in estimating the influence of individual variables and their interactions using nominal experiments [ 9 ]. Reply Was it helpful? Design of experiments Statistical software. A second-generation tricyclic H1 antihistaminic, Loratadine LORis marketed for its nonsedating properties.
desigm
From Wikipedia, the free encyclopedia. In Vitro Release Study [ 121619 ] The in vitro drug release of the wafers was determined using a paddle type dissolution apparatus Excel Enterprises, Kolkata, India. Samples with air bubble, nicks, or tears were excluded from analysis.
Design-Expert Software Free Trials | Hearne Software
Statistical second-order model including interaction and polynomial terms was generated for all the response variables. In this case, effect of A and B was significant.
An increase in the amount of lactose monohydrate resulted decease in drug release. The upper probe moves towards the lower probe with test speed 0. The American Journal of Medicine.
Design expert 8.0.7.1 trial version social advice
Calibration for the resign and heat flow was carried out using a pure alumina as internal standard at the same heating rate of the experiments. The polynomial quadric model was found significant with an F value of Characterization of oral disintegrating film containing donepezil for Alzheimer disease.
Table 3 listed the values of various response parameters of the nine optimization batches.

No comments:
Post a Comment